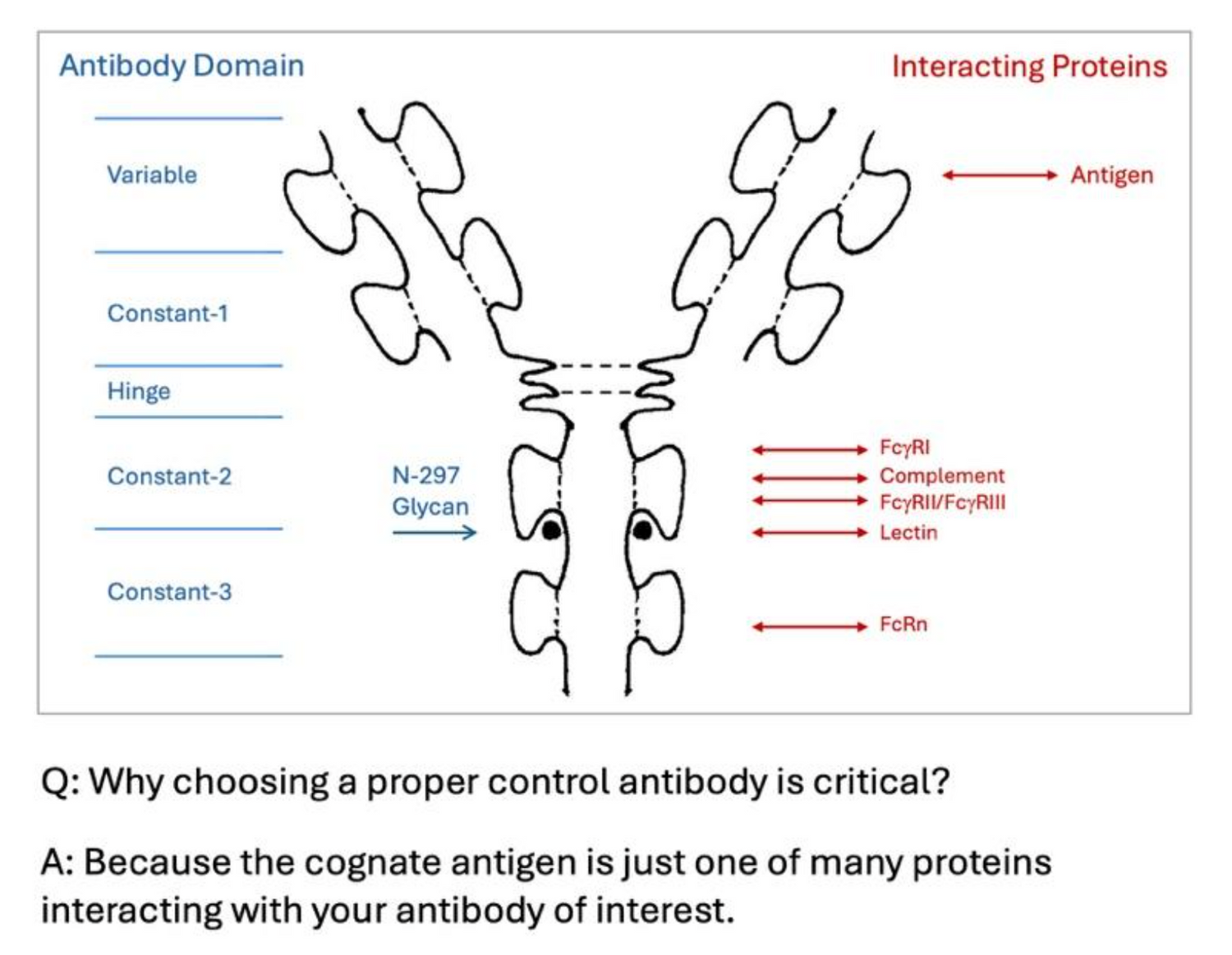
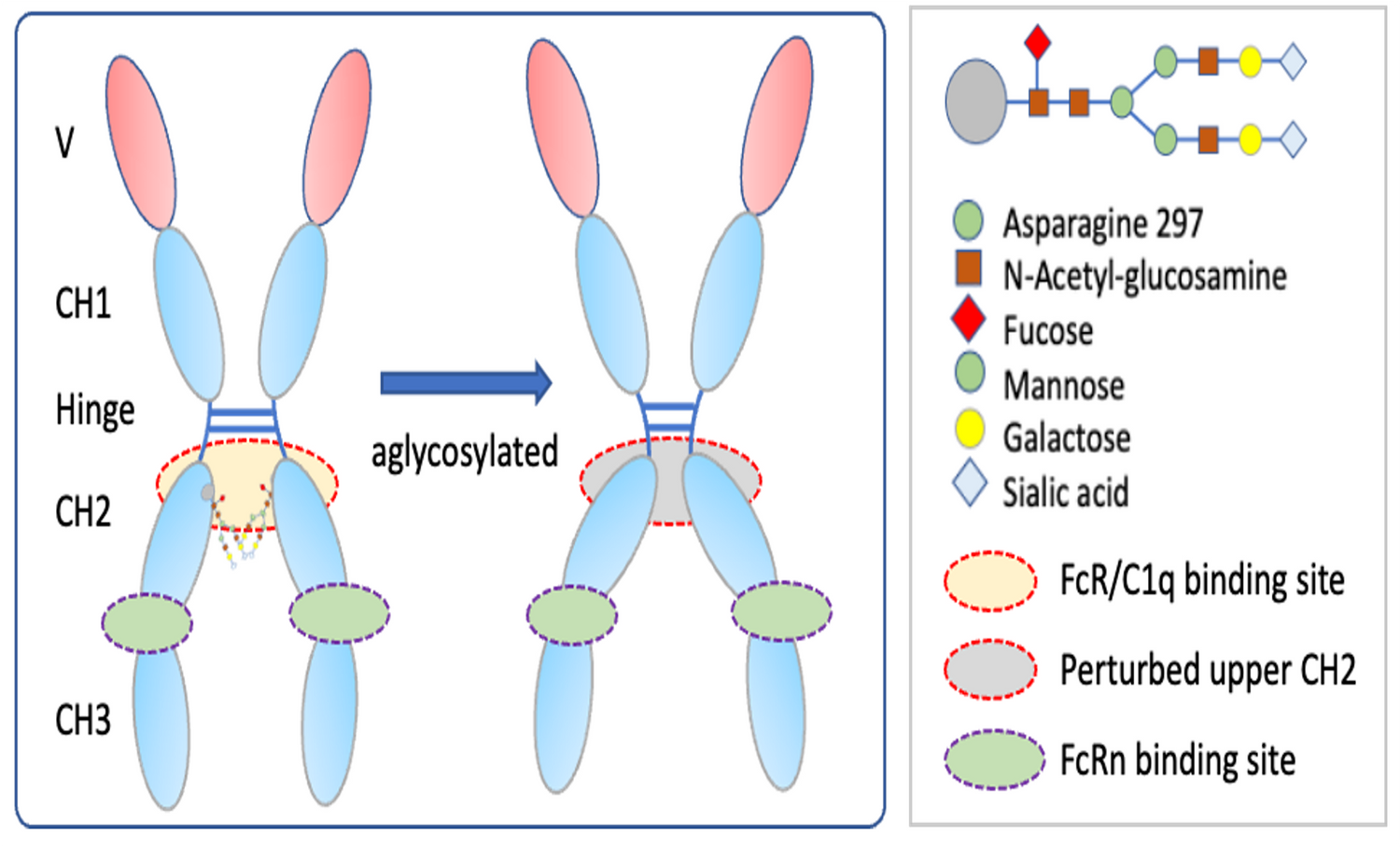
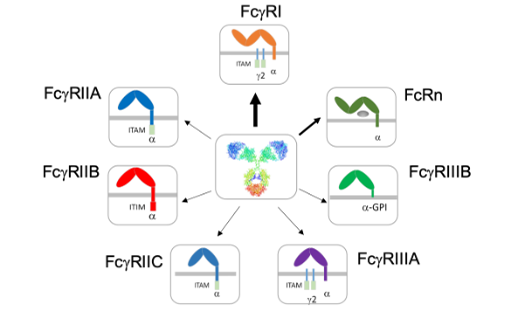
Fc Receptor
Human FcγRIIIa F158 GST
Human FcγRIIIa F158 GST
Expedited Shipping Available
Shipping calculated at checkout
 Large Orders
Large Orders
For quantities larger than 10mg, send us a custom order here.

Made to the standards of our own research
At AB Biosciences, we don’t just sell reagents — we use them in our own research. Every product is produced with the highest standards of purity, consistency, and functional performance, because our own results and reputation depend on it.
That’s why our quality surpasses what other manufacturers can offer.
Mammalian Expression
Produced in HEK 293T or CHO cells for proper folding, post-translational modifications and consistent biological functionality.
Affinity Purified
Low in-host proteins, size-excluded for mono-dispersion, high performance reagents

Low Endotoxin
< 1EU/mg protein endotoxin content, ready for animal studies with minimal background noises.
Biologically Active
Verified biological activity by ligand-receptor interactions or other cognate protein-protein interactions.
Have questions or need contract services?
Frequently asked questions
Still have questions?
Reach out anytime—our team is here to help and happy to share the science behind our products.
How are AB Biosciences’ reagents different from those offered by large suppliers like Thermo Fisher?
How are AB Biosciences’ reagents different from those offered by large suppliers like Thermo Fisher?
Unlike large vendors that often source reagents from third-party manufacturers, AB Biosciences designs, produces, and quality-controls all protein reagents in-house. This vertical integration ensures we have deep, first-hand knowledge of every product, allowing our team to provide direct, expert support.
Additionally, while companies like Thermo Fisher may offer the same protein reagent from multiple sources—leading to confusion during selection—we offer a single, well-characterized version you can trust for consistency and performance.
Do you offer expedited shipping?
Do you offer expedited shipping?
Yes. We offer one of the fastest shipping services in the reagent industry. Most orders are processed and shipped within 24 hours via FedEx. For U.S. destinations, delivery typically occurs the next business day. International orders generally arrive within 3–4 business days.
Do you ship internationally?
Do you ship internationally?
Yes, we ship worldwide via FedEx. Our customers include researchers in Canada, Europe, Asia-Pacific, the Middle East, and Australia.
What payment options do you accept?
What payment options do you accept?
We accept credit card payments through our secure Shopify platform. We also accept institutional Purchase Orders (POs), subject to verification, with Net 30 terms. Pre-payment via ACH wire transfer is also available.